Steel has many advantages, but its great disadvantage is the lack of corrosion resistance. Under the influence of Oxygen (O2) and Water (H2O), rust forms on the steel surface. The knife rusts in your pocket!

Fig. 1 Rust on steel [2]
The element that most strongly affects the corrosion resistance of steel is Chrome (Cr). With the increase of its content (starting from 3%) the resistance of steel to the formation of rust on its surface increases.
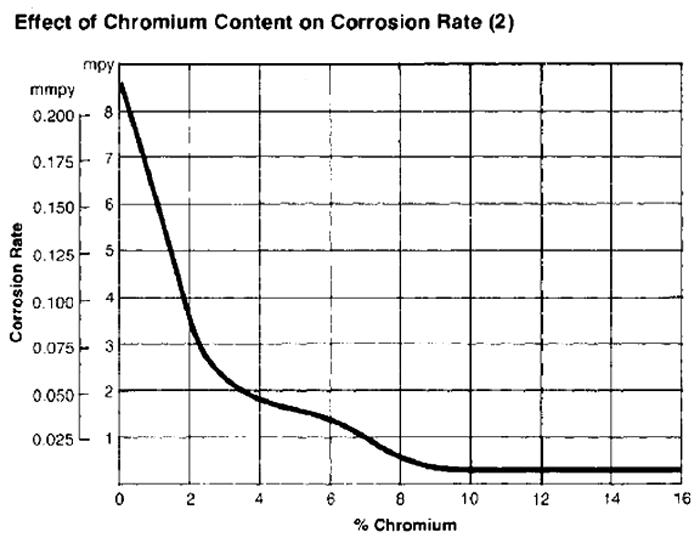
Fig. 2 Effects of Chromium content on corrosion speed in steel [2]
Precious metals are metals which do not corrode well under normal conditions (actually do corrode at all). Steel with more than 12% chromium behaves like precious metals! Then a thin, colourless, hard layer of Chromium Oxides forms on the surface of the stainless steel (>12% Chromium), which protects the steel against corrosion. It is sometimes called a passive layer,
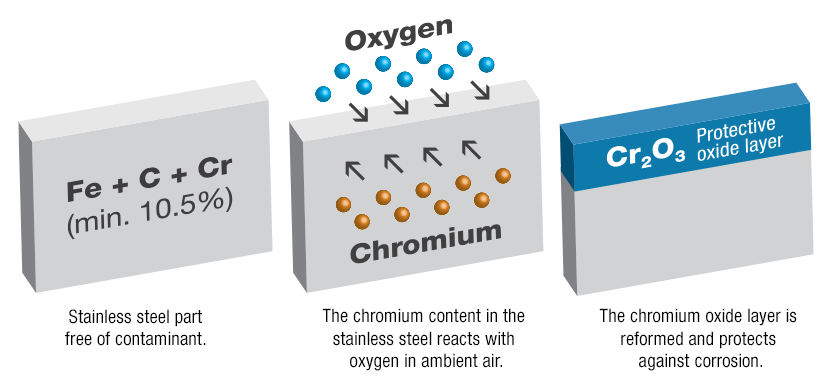
Fig. 3: Creation of a passive layer on stainless steel [3]
It is important to notice that it forms spontaneously on the steel surface and has an important property: it regenerates itself after mechanical damage!
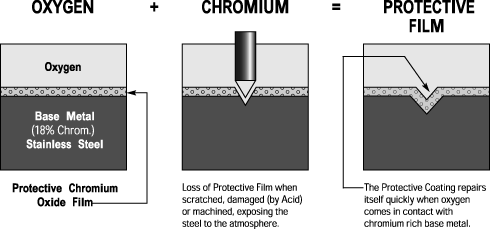
Fig. 4 Regeneration of damaged passive layer [4]
The standards state that steel with 10. 5% chromium is stainless steel. This is true, but in structural steels, on balustrades or in architecture. In stainless tool steel, Carbon (C) contained in steel "steals" part of Chromium, forming Chromium Carbides. It turns out that steel with a content of 11% Chromium, has this element to protect against corrosion with only 7%, the rest is "trapped" in Carbides, for example: steel D2 (AISI). D2 steel is therefore not a stainless steel, even though it meets the definition! That is why in stainless steel for knives (with carbon content up to 2%!) the content of Chromium is increased to over 14%, and in case of very high carbon content and high corrosion protection requirements to over 20%.

Fig. 5 Corrosion on D2 steel, knife lying only for half a year in the house! [5]


Fig. 6 and 7 Corrosion on D2 steel, the knife was immersed for 2 days in slightly saline water! [6]
Unfortunately, stainless steels are not ideal, although they do not cover up with rust, there is a different type of corrosion in them. During heat treatment, the steel may be exposed to Intergranular Corrosion (IPCC) if adverse factors accumulate. At this point, chromium carbides (Cr23C6) are formed in steel at the grain border, which "steal" the chromium from the surrounding area, reducing the content of this element in the surrounding area to below 12%. A galvanic cell is formed, the steel “eats” itself in the presence of Water (even that from the air)! As a result, "leprosy"J appears in the steel, the grains fall out of the steel, separated from the steel by corrosion.
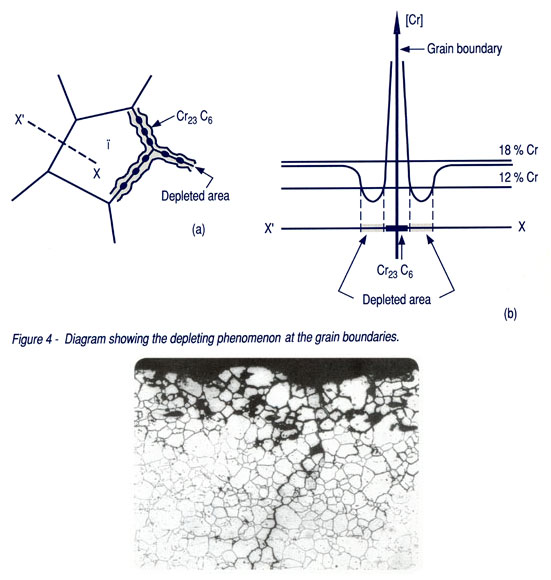
Fig. 8: Intercrystalline corrosion, scheme [7].

Fig. 9: Intercrystalline corrosion, knife [8]
Fortunately, this phenomenon is known to manufacturers of knives in Poland, who also know how to protect steel against intergranular corrosion.
They are constantly for tools resistant to intergranular corrosion (they are not stainless steels), they corrode, but very slowly, it is almost stainless steel (semi stainless). They are characterized by a content of Chromium above 5%, preferably 7-9%. They are rusty over time (for very, very, very, very long), and this rust also looks nice on them, like patina.
Bibliography
[1] https://mlodytechnik.pl/eksperymenty-i-zadania-szkolne/chemia/5381-korozja-metali
[2] https://vacaero.com/information-resources/vacuum-brazing-with-dan-kay/1459-321-stainless-steel-is-it-a-good-choice-for-brazing.html
[3] https://www.walter.com/surfox/passivation
[4] http://www.jamesglen.com.au/finishes-and-coatings/
[5] http://knives.pl Picture by Pitt
[6] http://knives.pl Picture by Escorpio
[7] http://www.christoforidis.gr/en/intergranular_corrosion.php
[8] http://www.amteccorrosion.co.uk/stainlesssteel.html