Banally, pollution plays a very important role right after coal. WHY? Because if there is too much of it, even adding the entire Mendeleyev table will not make the steel suitable for making knives out of it.
Polluters in steel are Sulphur (S), Phosphorus (P), Hydrogen (H), Oxygen (O), Nitrogen (N). The presence of these elements (together and separately) increases the tendency of steel to fragile crack, and nobody wants their knife to crack, right?
Harmful admixtures are removed during the steel production process, the less of them the better the steel is. The more of them can be removed, the less likely the steel is to crack. If the steel is very clean, the manufacturer boasts about the fact from left to right. If he does not boast this means that the steel is of normal quality.
The measure of ductility in steel is the impact strength. The higher the impact strength of the steel, the more resistant the knife will be to cracking. The impact testing method is the Charpy test. [1]. The breaking work value is temperature dependent. At 50O C dirty steel (lots of Sulphur and Phosphorus) can have a high impact strength. However, no one buys knives to use in Africa, in many parts of the world the temperature drops well below 0O C! A sudden reduction in impact strength at a given temperature is called the brittleness temperature. The lower the brittleness temperature, the more confidence you have in your knife, the more resistant it will be to cracking!
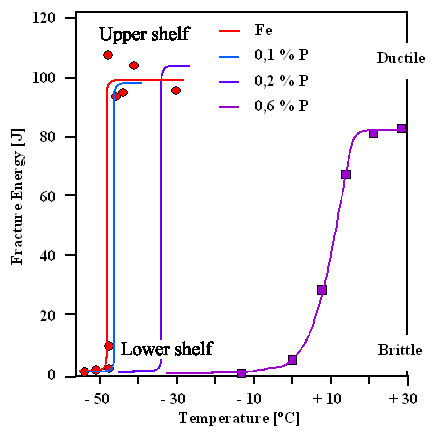
The most important polluters in steel are Sulphur and Phosphorus and their content should be below 0. 03% and less.
Fig. 1 Influence of Sulphur content on impact strength in steel [2]
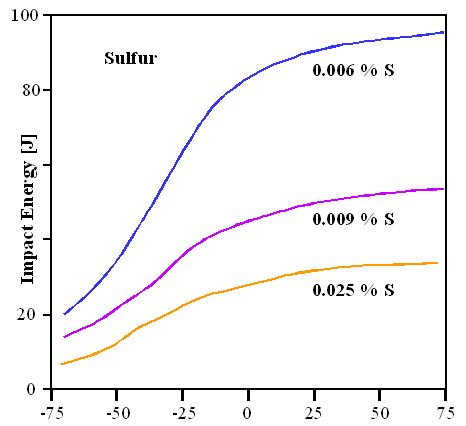
Fig. 2 Influence of Phosphorus content on Steel impact strength [2]
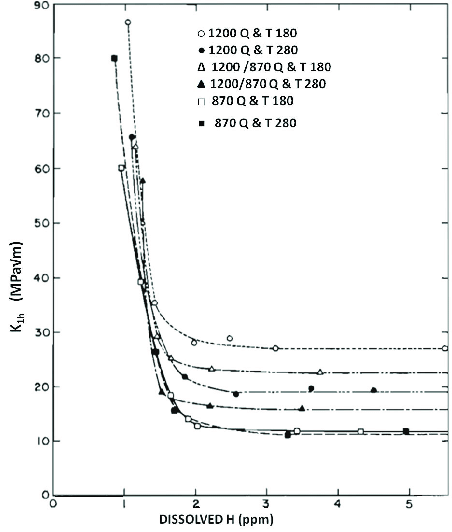
Oxygen and nitrogen are removed at the steelworks, as are Sulphur and Phosphorus. A strange and dangerous element is Hydrogen, because it can be injected into steel while making knives! And a small amount of it causes the steel to crack at positive temperatures! Heating for forging and hardening in burning furnaces fired with gas, coal, coke, charcoal and oil is particularly dangerous. Heating in electric furnaces does not affect the hydrogen content of steel, well… it actually does: it always lowers the Hydrogen content! Fortunately, most of the Polish knife manufacturers who forge and harden their knives in furnaces which are fueled with solid, liquid and gaseous fuel know about this fact! And they also know how to remove Hydrogen into steel, as well as how to remove it if it gets there.
Fig. 3 Impact of hydrogen content on impact strength of hardened and tempered steel 4340 (AISI) [3].
Bibliography:
[1] https://en.wikipedia.org/wiki/Charpy_impact_test
[2] https://www.tf.uni-kiel.de/
[3] Guido Zucha, Jacopo Tirillò, A. Mocci, M. Bernabei, F. De Paolis: „Hydrogen Embrittlement and Fatigue Fracture of a Crankshaft of an Internal Combustion Engine”. XXIII Italian Group of Fracture Meeting 2015.
Powered by MELONTOOLS