In steel besides Carbon (C) (and of course Iron (F)!), polluters, we can also observe the occurrence of alloy elements which improve the properties of the steel. These are the reasons why alloy steels are better than a pure alloy of iron with carbon.
The first group consists of admixtures added when pouring steel into steel vats (from a steel furnace). They bind the Oxygen (O) contained in the steel. Manganese (Mn), Silicon (Si), Aluminium (Al)-- It is thanks to them that we do not have to worry about too much oxygen in steel! Manganese and Silicon are added to liquid steel in the form of Ferro-alloys, and Aluminium is thrown into the metallic form (in " ingots").

Fig. 1 Iron-manganese [1]

Fig. 2 Ferrosilicon [1]

Fig. 3 Ingot aluminium [3]
Manganese, in addition to oxidation, has another important function to fulfill : it binds the sulfur (S) in steel. Unfortunately, there is always sulphur in steel, sometimes little, sometimes very little, but it cannot be removed completely. Sulphur with Iron forms a low-melting Iron Sulphide (FeS), with a low melting point of 1195O C . However, even at temperatures above 980 °C it becomes soft and ductile. There would be nothing dangerous about this, but the steel hammerings and rollings at temperatures higher than 1000O C, if the steel contains iron sulfide the steel breaks down (!) and is suitable only for scraps-metals. Increased amounts of Manganese bind Sulphide in Manganese Sulphide (MnS) with a melting point of 1620O C, at typical (and even higher) steel rolling temperatures it does not impair the properties of the steel.
In addition, Manganese increases the hardenability of the steel, increases the impact resistance at low temperatures. However, its content in tool steels should not be above 1. 8%, because when it gets above this temperature paradoxically it lowers the breaking work of the steel.
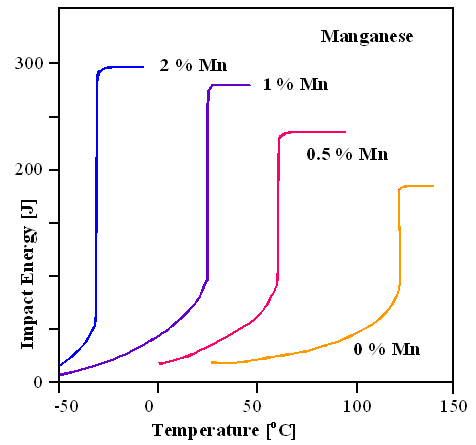
Fig. 4 Impact of Manganese on steel impact strength [3].
Silicon, in addition to oxygen binding, affects the elasticity of hardened steel, hence its increased amount is often found in spring steels. It also increases the hardenability, although less than Manganese. Usually 1%, maximum 2% of it can be found in the steel for knives.
Aluminium is the most underestimated element in carbon steels used for knives. In addition to oxygen binding, it indirectly affects the impact strength of steel, creating Aluminium Oxide (Al2O3) and Aluminium Nitride (AlN) , which resist grain growth at high temperatures. They make the steel finer-grained. The smaller the grains in the steel, the higher the impact resistance. Aluminium reduces the grain size in carbon steels and increases the impact resistance!
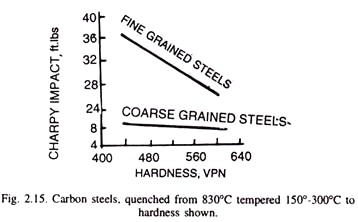
Fig. 5 Impact of grain size on impact strength in steel [4]
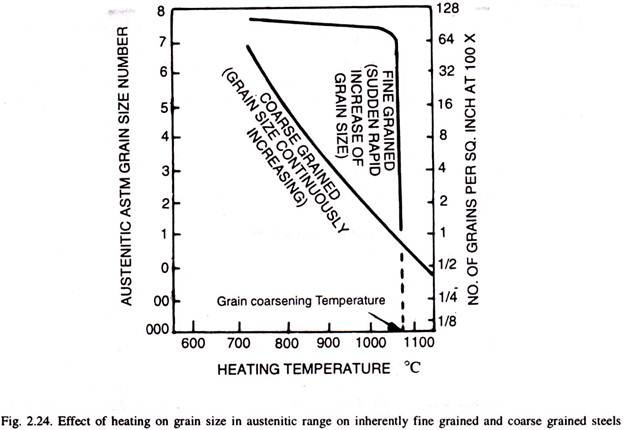
Fig. 6 Difference in grit size between fine-grained and coarse-grained steels [4]
In most carbon tool steels used for knives in Poland, aluminium is in sufficient quantity to ensure that the steel in the knives it has a small grain and has a high impact strength.
Bibliography:
[1] http://www.redbor.pl/encyklopedia/uklad_okresowy/zelazo.htm
[2] http://www.jap.cz/pl/pozostale-produkty/surowce-i-polfabrykaty-hutnicze/aluminium-do-odlewania-stali/
[3] https://www.tf.uni-kiel.de
Powered by MELONTOOLS